WELCOME TO FARM LOYIHA
We help pharmaceutical and biotech companies comply with
international quality and safety standards, building trust and
confidence with clients worldwide.
Why Choose Farm Loyiha?
Expertise in pharmaceuticals and biotechnologyy
Full-cycle support: from startup to global operations
Tailored solutions for every business stage
Quality Compliance Consulting
We ensure your company meets all international quality and safety requirements with confidence.
Regulatory Strategy & Support
From documentation to audits, we guide you through every step of regulatory approval.
Efficient Project Solutions
Customized strategies that help reduce risks, optimize processes, and achieve faster market entry.
About Us
We are a team of experts in GxP (GMP, GDP, GCP, GLP) standards, assisting pharmaceutical and biotechnology companies in achieving compliance with international quality requirements. Our expertise spans the entire cycle: from developing documentation and SOPs to preparing for FDA and EMA inspections. The company's mission is to enhance trust in our clients' products and ensure their safety for patients worldwide.
-
Comprehensive Expertise
Decades of experience in regulatory compliance, documentation, and international audits.
-
Tailored Solutions
Customized strategies designed to meet the unique needs of each company.
-
Trusted Partner
A reliable partner supporting your success in global markets and inspections.
Specialists
Departments
Research Labs
Awards
Services
Necessitatibus eius consequatur ex aliquid fuga eum quidem sint consectetur velit
Pharmaceutical Facility Design
Design of pharmaceutical and dietary supplement (DS) production plants, quality control laboratories, storage, and distribution facilities in compliance with international standards.
Technical support
Development and implementation of tailored technical solutions, considering the specific production direction of each company.
Supply of Raw Materials and Components
Procurement and delivery of high-quality raw materials and components for pharmaceutical and DS manufacturing.
New Product Launch
Establishing serial production of new formulations for local pharmaceutical manufacturers.
Technology transfer
Introduction of complex, high-tech production processes from foreign companies into local manufacturing.

Contract Manufacturing
Hic molestias ea quibusdam eos. Fugiat enim doloremque aut neque non et debitis iure. Corrupti recusandae ducimus enim.

Registration and Documentation
State registration of local and foreign medicines, medical devices, and equipment in accordance with national regulations.
Implementation of GxP Standards
Development and integration of GxP quality standards (GMP, GDP, GCP, GLP) into local manufacturing processes.

Audits & Training
Conducting audits of foreign manufacturers based on Uzbekistan’s national standards, preparing for official inspections, and organizing professional training programs for pharmaceutical specialists.
Departments
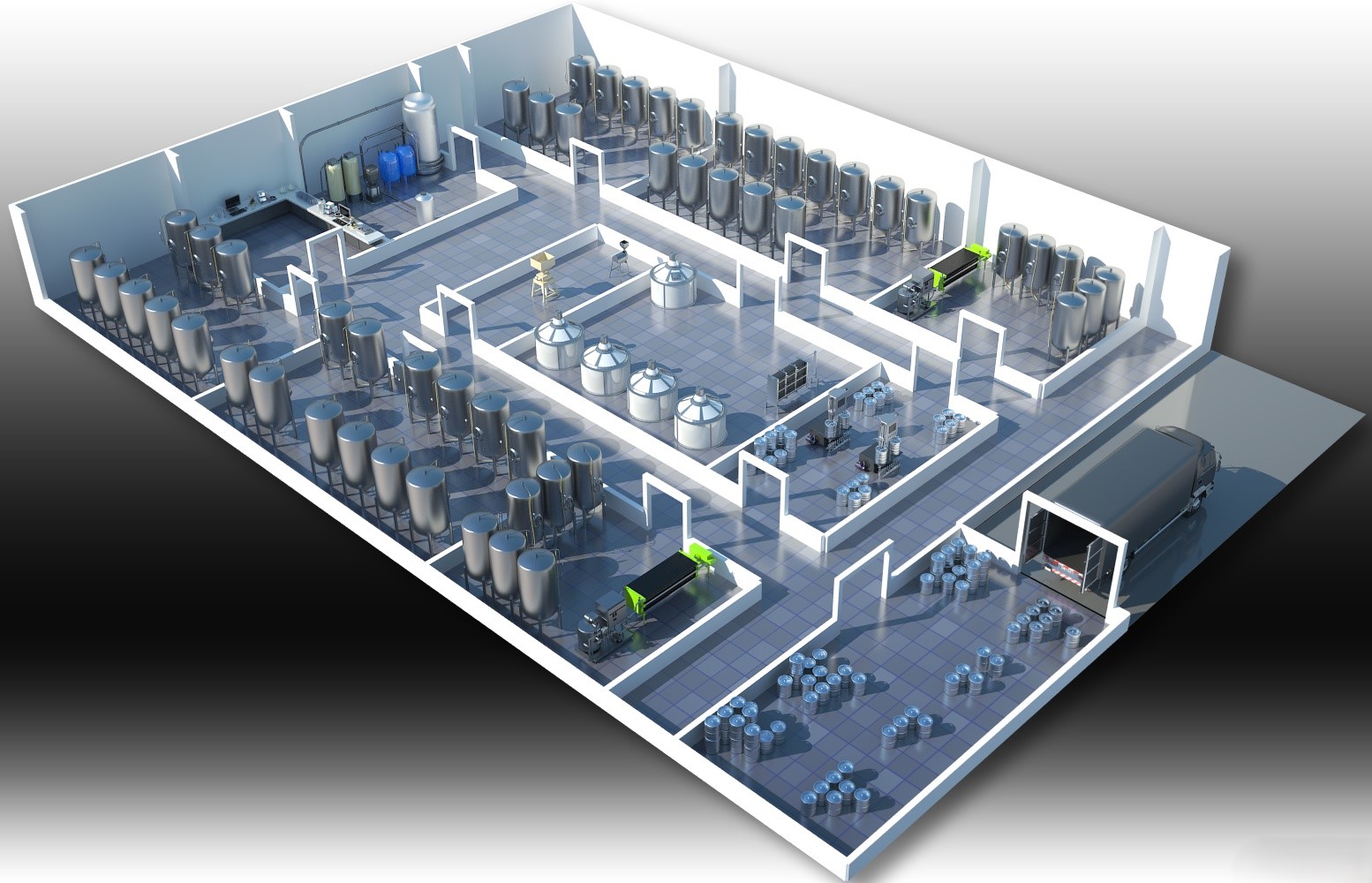
Pharmaceutical Facility Design
We design pharmaceutical and dietary supplement (BFQ) facilities in line with international standards – from production workshops and quality control laboratories to storage and distribution centers.
- GMP-compliant cleanroom classification
- Contamination risk minimization
- Water, air, and HVAC system planning
- Utility and emergency exit layouts
Technical Supply & Equipment
We provide end-to-end technical support for pharmaceutical manufacturers:
- Supply and installation of production & packaging equipment
- HVAC and water system implementation
- Delivery of laboratory devices, reagents, and consumables

Raw Material & Packaging Supply
We source and deliver high-quality raw materials and packaging solutions from leading international suppliers, tailored to your production needs.

New Product Development
We assist in launching new pharmaceutical and dietary supplement formulations, including:
- Technology transfer of complex products
- Scaling lab technologies to industrial production
- Staff training for new production lines

Contract Manufacturing
We provide contract-based industrial-scale production of pharmaceuticals and supplements:
- Tablets, capsules (hard & soft), ointments, creams, gels, suppositories
- Syrups, suspensions, extracts, tinctures, and solutions
- Full regulatory documentation and certification support

Regulatory Affairs & Registration
We prepare and submit complete regulatory dossiers for state registration of pharmaceuticals, medical devices, and materials, ensuring compliance with all national requirements.

GxP Implementation
We help companies implement GxP standards in Uzbekistan:
- GMP, GDP, GLP, GSP, and GPP compliance
- ISO standards integration
- Documentation systems and staff training

Audit & Inspection Preparation
We conduct audits for foreign manufacturers and prepare them for official inspections in line with Uzbekistan’s national standards.
- On-site and online assessments
- Facility and system evaluations
- Documentation review and compliance gap analysis

Training & Capacity Building
We organize professional training programs for pharmaceutical industry staff:
- Practical and theoretical training for production, QC labs, and storage facilities
- Continuous support and certification upon completion

Disinfection & Cleaning Solutions
We supply custom-made disinfectants and cleaning equipment for:
- Pharmaceutical and medical device manufacturers
- Distribution warehouses and pharmacies
- Hospitals, clinics, and laboratories

Frequently Asked Questions
Which standards do you work with?
Our services are carried out in accordance with national and international GxP (GMP, GDP, GLP, GSP, GPP) and ISO standards. We also conduct audits at manufacturing companies abroad based on the requirements of international regulatory authorities. In addition, we develop recommendations and corrective measures to align processes and documentation systems of local and foreign pharmaceutical enterprises with current legislation and standard requirements.
Do you only work with pharmaceuticals?
No. We provide services to manufacturers of pharmaceutical products, dietary supplements, medical devices, and medical equipment.
Do you supply equipment and raw materials?
Yes. We supply production and packaging equipment, laboratory instruments, reference standards, reagents, and high-quality raw and packaging materials.
Can you help us launch new products?
Absolutely. We not only develop technologies for generic products but also for new formulations. We handle technology transfer and provide training for company staf.
Do you only design facilities, or do you also support production?
We provide end-to-end services: facility design, equipment installation, supply of raw materials, preparation of documentation, and full-scale production launch.
Do you provide regulatory registration support?
Yes. We prepare the complete regulatory dossier, submit it to the competent state authorities, and ensure full support for official registration within the required timelines.
Do you offer training for employees?
Yes. We organize theoretical and practical training programs for production, laboratory, and warehouse staff. Upon completion, participants receive certificates of compliance.
Are your services only available in Uzbekis
No. We work with both local and international pharmaceutical companies, helping them achieve compliance with global standards.
Testimonials
Ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident.
Gallery
Necessitatibus eius consequatur ex aliquid fuga eum quidem sint consectetur velit
Contact
Necessitatibus eius consequatur ex aliquid fuga eum quidem sint consectetur velit
Location
Mirzaabad St. 42, 100000, Tashkent, Uzbekistan
Call Us
+1 5589 55488 55
Email Us
info@example.com
 EN
EN RU
RU UZ
UZ